Research Monograph for National Policy on Implants
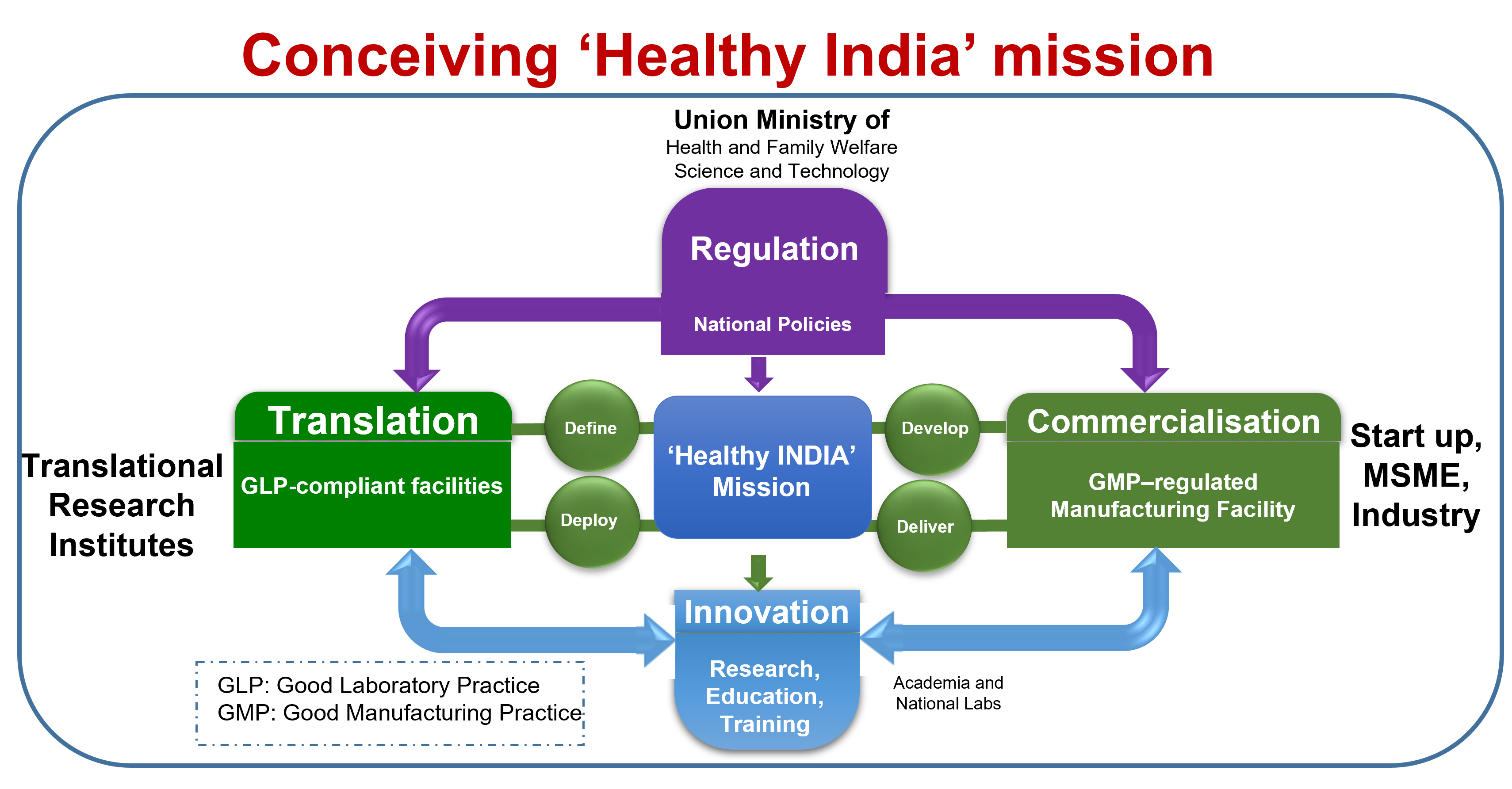
This monograph presents the following policy-related recommendations, to stimulate accelerated journey of scientific discovery, together with manufacturing of next generation biomaterials and implants in developing nations, with India as a model country.
- Implement a framework to streamline accelerated regulatory approval mechanisms.
- Strengthen basic research for accelerated innovation of next-generation biomaterials
- Build up context-driven, translational research toward the treatment of human diseases.
- Nourish industry collaborations and capabilities, grow and sustain start-ups
- Revamp medical education and clinical research ecosystem to train clinicians
Implement action plans to harness benefits of biomaterial implants for Public healthcare
Science to Technology and Products: Biomaterials and Biomedical Engineering
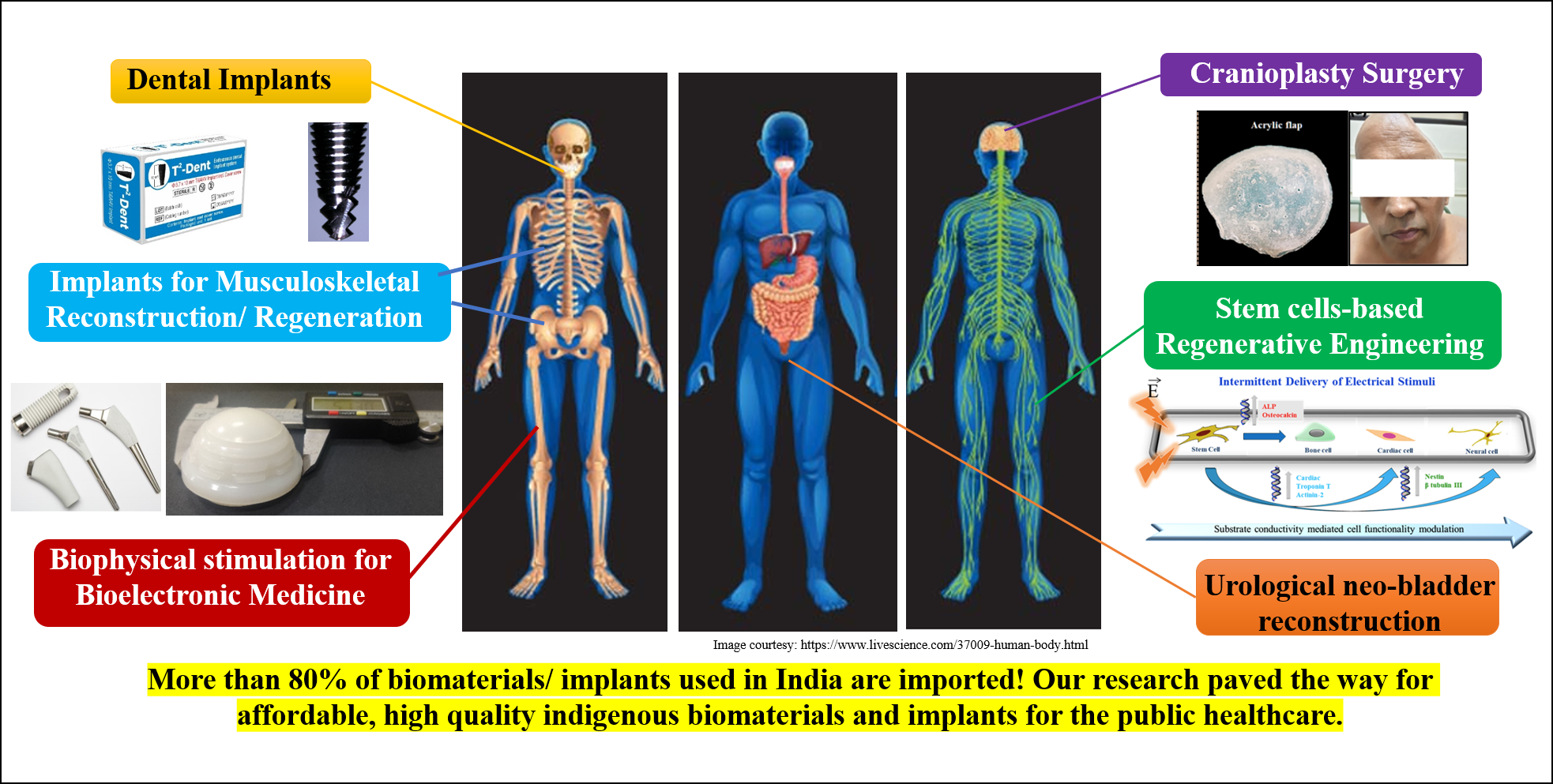
Biophysical stimulation for regenerative engineering and to combat implant-associated infection
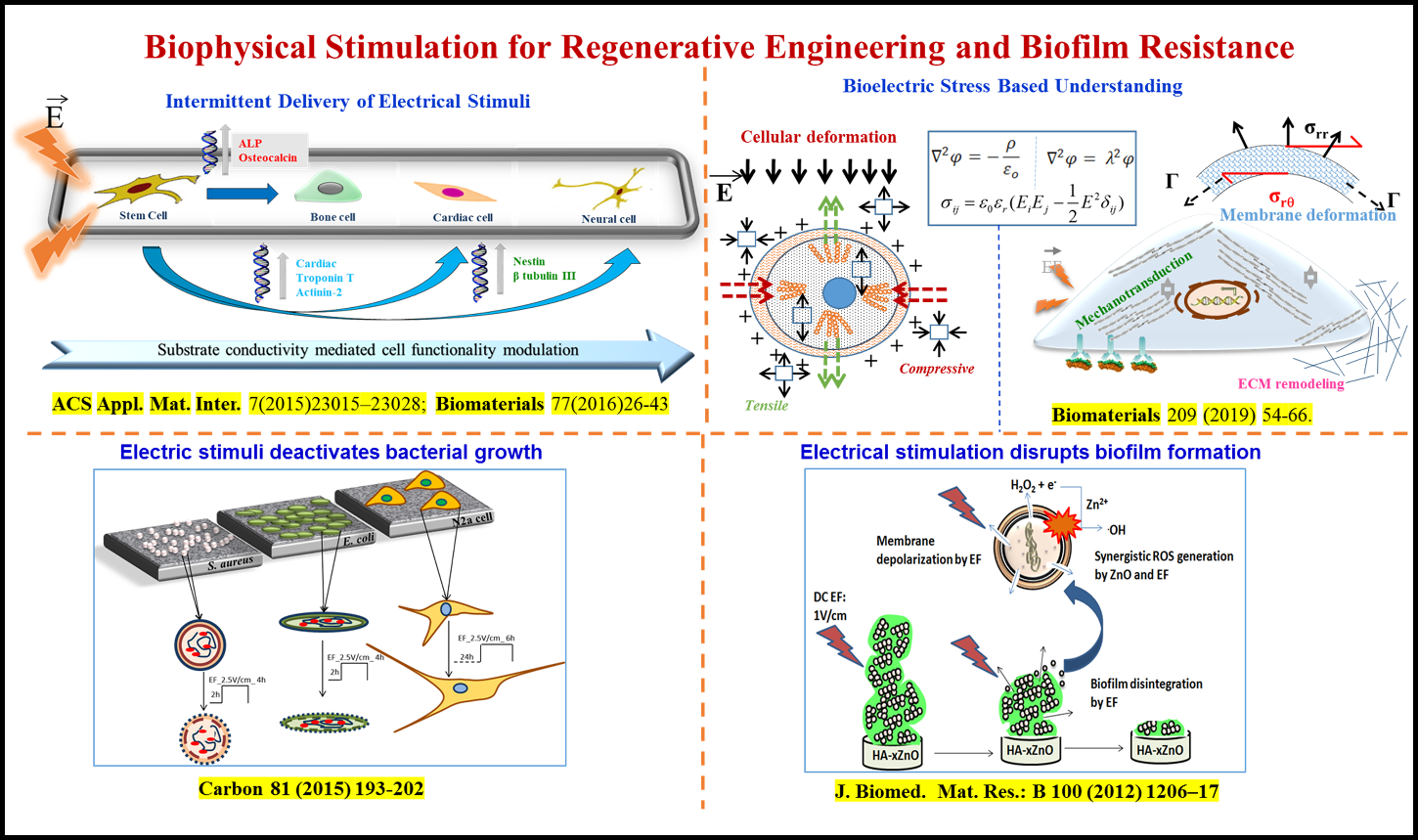
Bikramjit’s innovation at the intersection of biomaterials design, electrical and magnetic stimulation, has enabled us to understand the biophysical origin of tailored electrical stimuli-directed differentiation of bone marrow-derived stem cells into bone, cardiac, neural/glial-like cells with clinically desired electrophysiological functionality. This has been validated on elastically stiff/compliant biomaterials (HA-CaTiO3, HA-BaTiO3, doped PANI, PVDF-CNT-BaTiO3, etc.) under 2D culture and/or in biomicrofluidic devices under dynamic culture conditions. He has convincingly shown that electrical stimuli can inhibit cell proliferation, while promoting early cell differentiation, in vitro. His research team provided insights into the impact of bioelectric stresses on cellular deformation and mechanotransduction, using mathematical modeling. His research discovered that the intermittent delivery of magnetic stimuli drives osteogenesis of human mesenchymal stem cells on magnetoactive biomaterials.
In the context of implant associated infection, hydroxyapatite composites reinforced with Fe3O4 and ZnO were designed to induce bacteriostatic/ bactericidal effect against antibiotic-resistant strains (e.g. MRSA). His group is the first to demonstrate key role of adjuvant treatment approaches, e.g. external biophysical stimulation (electric and magnetic stimuli), to enhance the bactericidal or bacteriostatic properties on implantable biomaterials (see figure below). Together with endodontists, his team illustrated the critical role of pulsed magnetic stimuli to restrict the growth of E. faecalis strains, commonly implicated in root canal therapy treatment. He is currently translating in vitro outcomes to implantable miniaturized microelectronic modules for applications in bioelectronic medicine. This body of work has profound significance on the bioengineering strategies for bone, neural and cardiovascular applications, while combating implant-associated infection.
3D Binderjet printing of implantable biomaterials and cells (3D Bioprinting)
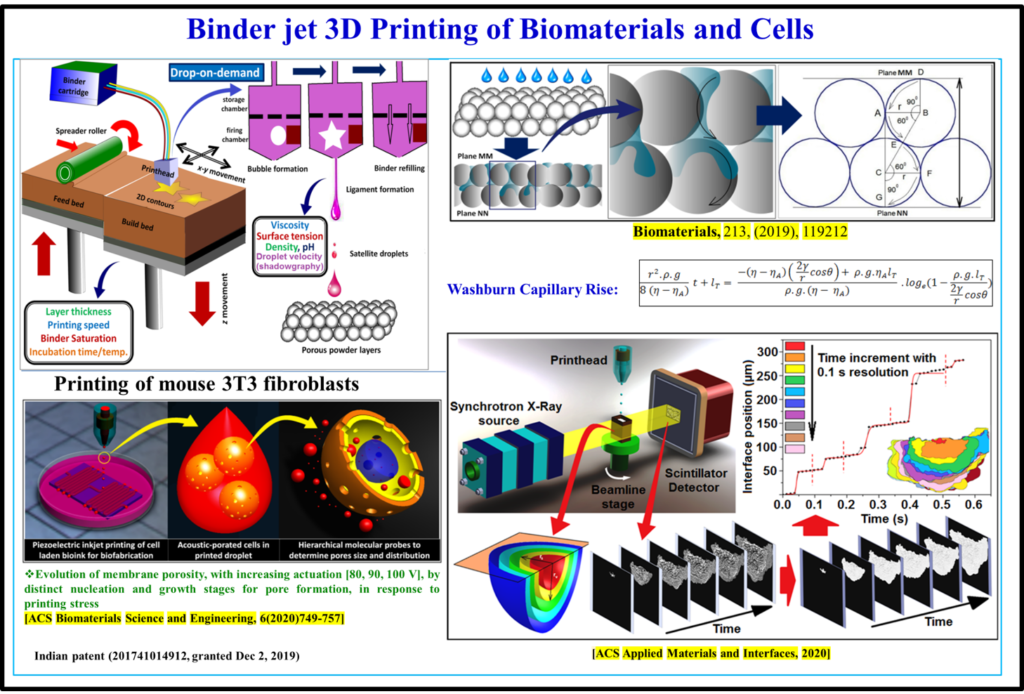
In the field of additive manufacturing, laser or electron beam–based 3D printing is widely investigated for manufacturing designed materials. These variants of additive manufacturing do not however allow printing of biomaterials or living system components (proteins, cells) in physiologically relevant process conditions. Bikramjit’s research group developed a quantitative understanding of the process physics of 3D binderjet printing of biomaterials. Using a carefully planned set of printing experiments, his research has validated the formulation of an in situ polymerizable acrylic binder in processing implantable Ti6Al4V, calcium phosphate and Mg-Sr-phosphate biomaterials with highly interconnected porosity, acceptable strength reliability and biocompatibility. Recently, a high throughput time-resolved X-ray imaging study was carried out to quantitatively analyze the spatio-temporal evolution of wetting contours in the powder bed during infiltration. Bikramjit’s team has successfully extended the Denesuk/Washburn model to rationalize the experimentally determined kinetics of binder infiltration into the powder bed. His research group also proposed the critical role of actuating voltage on membrane poration during piezoelectric 3D printing of connective tissue cells using molecular probes. 3D printing has been successfully adapted for manufacturing of 3D models for the cranioplasty surgery application.
Biomaterials and implants for musculoskeletal reconstructive applications
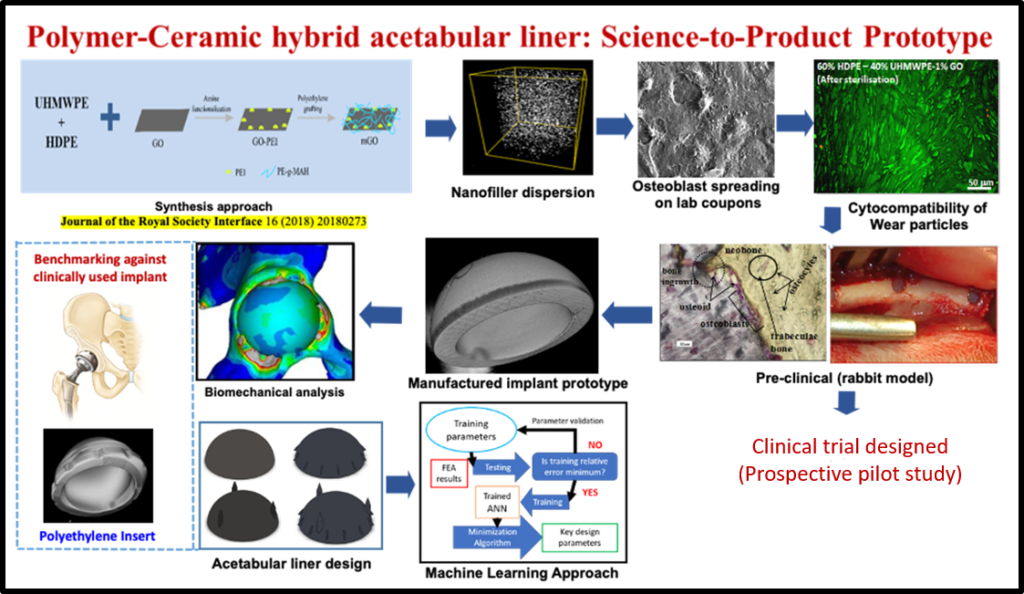
According to a published report of the World Health Organization (WHO), about 190 million adults suffer from osteoarthritis and related disabilities worldwide. This is commensurate with the fact that the number of revision surgeries has increased at about the same rate as the number of primary surgeries of joint replacement due to prostheses failures. In a decade-long research program, his research group made important breakthroughs in developing three different generations of polymer-ceramic hybrid acetabular sockets, namely HDPE-HA-Al2O3 hybrid composites, polyethylene grafted graphene oxide (GO) reinforced high density polyethylene (HDPE) composites and lately, a UHMWPE-HDPE blend with surface modified GO reinforcement. For example, a new synthesis approach was developed to chemically couple GO in polymer blends, which resulted in high mechanical strength (~65 MPa) and wear resistance properties with acceptable biocompatibility. The scalability for high volume manufacturing of acetabular liners (44, 46 or 48 mm outer diameter with 8 mm wall thickness) with acceptable surface finish has been established. The new implant design was accomplished by biomechanical analysis of principal stresses in periprosthetic bone around the acetabular joint and current efforts are underway to adopt machine learning algorithms to accelerate the implant design. The augmented bone tissue regeneration around the variants of hybrid composite were demonstrated in both cylindrical and segmental defect models in femurs of experimental rabbits for a period of up to 26 weeks. This work has entered into a clinical study, which has been conceived to evaluate the safety of the new generation acetabular liners in patients undergoing hip replacement surgery.